Research
Our lab studies how cells communicate with one another, a process that regulates nearly all aspects of animal development and, when damaged, leads to many diseases including cancer. Transmembrane proteins are the essential factors that transmit fate signals from the cell's external environment to its interior. In this regard, transmembrane proteins serve as gatekeepers whose initial decisions ultimately determine a cell fate pathway’s final activity state. Surprisingly, we still do not understand how transmembrane proteins are regulated to produce proper amounts of downstream signals at the right times and places within the organism. This has limited our ability to control cell fate signaling in many diseases including cancer. The Myers lab is studying the biochemical and biophysical mechanisms that regulate transmembrane protein activity in cell fate pathways. A better understanding of these processes will help us to therapeutically manage birth defects, regenerative disorders, and malignancies.
Our lab studies how cells communicate with one another, a process that regulates nearly all aspects of animal development and, when damaged, leads to many diseases including cancer. Transmembrane proteins are the essential factors that transmit fate signals from the cell's external environment to its interior. In this regard, transmembrane proteins serve as gatekeepers whose initial decisions ultimately determine a cell fate pathway’s final activity state. Surprisingly, we still do not understand how transmembrane proteins are regulated to produce proper amounts of downstream signals at the right times and places within the organism. This has limited our ability to control cell fate signaling in many diseases including cancer. The Myers lab is studying the biochemical and biophysical mechanisms that regulate transmembrane protein activity in cell fate pathways. A better understanding of these processes will help us to therapeutically manage birth defects, regenerative disorders, and malignancies.
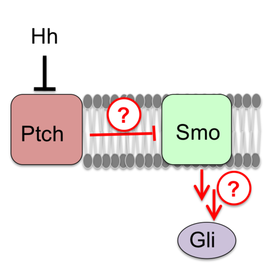
Hedgehog Signal Transduction
Much of our work focuses on the Hedgehog (Hh) pathway, a major player in development and regeneration that controls the formation of nearly every organ in our bodies. The Hh pathway is also a fascinating model for how membrane proteins and lipids influence cell fate decisions. Insufficient Hh signaling is linked to birth defects, while pathway overactivation drives several common cancers. Yet surprisingly, the critical biochemical events regulating Hh signaling at the membrane remain largely unknown. We are dissecting the biochemical processes that enable cells to respond to Hh signals. Our approach utilizes novel optical, biochemical, and electrical sensors to monitor key Hh signal transduction events as they unfold in real-time. By using these sensors to study key Hh pathway steps in both living and in vitro systems, we are gaining a deep understanding of the underlying transduction mechanism.
Much of our work focuses on the Hedgehog (Hh) pathway, a major player in development and regeneration that controls the formation of nearly every organ in our bodies. The Hh pathway is also a fascinating model for how membrane proteins and lipids influence cell fate decisions. Insufficient Hh signaling is linked to birth defects, while pathway overactivation drives several common cancers. Yet surprisingly, the critical biochemical events regulating Hh signaling at the membrane remain largely unknown. We are dissecting the biochemical processes that enable cells to respond to Hh signals. Our approach utilizes novel optical, biochemical, and electrical sensors to monitor key Hh signal transduction events as they unfold in real-time. By using these sensors to study key Hh pathway steps in both living and in vitro systems, we are gaining a deep understanding of the underlying transduction mechanism.
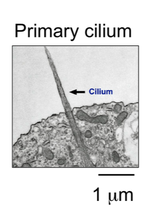
The Primary Cilium
Apart from its roles in development and cancer, Hh is a window into the intricate signaling processes that unfold within the vertebrate primary cilium. The cilium is a tiny antenna-shaped membrane appendage critical to the development and function of nearly all our physiological systems. Mutations in ciliary components affect numerous aspects of human physiology and can profoundly influence cancer progression. However, we know little about how ciliary dysfunction causes such effects. This is mainly because ciliary signaling pathways are often studied with indirect methods that are unable to capture the elaborately choreographed signaling events within this organelle. We are shedding light on this question by merging in vitro biochemical reconstitution assays with novel real-time optical and electrical readouts of ciliary receptor activity.
Apart from its roles in development and cancer, Hh is a window into the intricate signaling processes that unfold within the vertebrate primary cilium. The cilium is a tiny antenna-shaped membrane appendage critical to the development and function of nearly all our physiological systems. Mutations in ciliary components affect numerous aspects of human physiology and can profoundly influence cancer progression. However, we know little about how ciliary dysfunction causes such effects. This is mainly because ciliary signaling pathways are often studied with indirect methods that are unable to capture the elaborately choreographed signaling events within this organelle. We are shedding light on this question by merging in vitro biochemical reconstitution assays with novel real-time optical and electrical readouts of ciliary receptor activity.
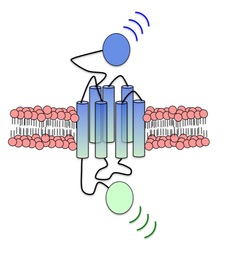
New Roles for Lipids and Kinases in Signal Transduction
Our work on Hh has led to new principles regarding how lipids can bind to and activate membrane receptors, and how membrane receptors can signal to kinases. We are now using these principles to unravel the signaling mechanisms in a variety of other signaling pathways, including G protein-coupled receptor cascades critical throughout human health and physiology. These studies will dramatically increase our understanding of many different health- and disease-relevant signaling pathways. They will also help us engineer better drugs to effectively control transmembrane protein activity while minimizing issues like drug resistance and side effects.
Our work on Hh has led to new principles regarding how lipids can bind to and activate membrane receptors, and how membrane receptors can signal to kinases. We are now using these principles to unravel the signaling mechanisms in a variety of other signaling pathways, including G protein-coupled receptor cascades critical throughout human health and physiology. These studies will dramatically increase our understanding of many different health- and disease-relevant signaling pathways. They will also help us engineer better drugs to effectively control transmembrane protein activity while minimizing issues like drug resistance and side effects.